Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Falyouna, O.*; Maamoun, I.; Ghosh, S.*; Malloum, A.*; Othmani, A.*; Eljamal, O.*; Amen, T. W. M.*; Oroke, A.*; Bornman, C.*; Ahmadi, S.*; et al.
Journal of Molecular Liquids, 368, Part B, p.120726_1 - 120726_25, 2022/12
被引用回数:5 パーセンタイル:42.74(Chemistry, Physical)Despite the carcinogenic and other adverse health effects ofchloramphenicol (CAP), it is frequently detected in different water sources (e.g., groundwater, surface water, wastewater effluents, etc.) due to ongoing, illegal, and abusive application of CAP in veterinary medicine. Although extensive research has been carried out to develop effective treatment technologies to remove the persistent CAP from aqueous mediums, yet there is no critical review of these studies to the best of our reach This review will be the first in the literature to comprehensively summarize the state-of-the-art treatment techniques for CAP removal from water. We report the removal of CAP by adsorption, biodegradation, nanoscale zerovalent iron technology (nZVI), and advanced oxidation processes (AOPs). The result shows that carbon-based adsorbents have more q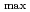 equal 892.86 mg/g for Porous carbon material from
equal 892.86 mg/g for Porous carbon material from 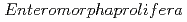 . The Langmuir- Freundlich isotherm and pseudo-second order kinetics model were reported to best describe the isotherm and kinetic model respectively. Removing the CAP via biodegradation would achieve the advantages of low operating costs, and environmental friendliness. The process of AOPs among the various treatment options can be a promising method for CAP degradation in water. This review comprehensively summarizes the state-of-the-art treatment techniques for CAP removal from water. Particularly, serving as an inclusive reference for future researchers to easily define the research gabs in the literature and plan for their future work in developing novel treatment methods to decontaminate CA-contaminated waters.
. The Langmuir- Freundlich isotherm and pseudo-second order kinetics model were reported to best describe the isotherm and kinetic model respectively. Removing the CAP via biodegradation would achieve the advantages of low operating costs, and environmental friendliness. The process of AOPs among the various treatment options can be a promising method for CAP degradation in water. This review comprehensively summarizes the state-of-the-art treatment techniques for CAP removal from water. Particularly, serving as an inclusive reference for future researchers to easily define the research gabs in the literature and plan for their future work in developing novel treatment methods to decontaminate CA-contaminated waters.
Idham, M. F.*; Falyouna, O.*; Eljamal, R.*; Maamoun, I.; Eljamal, O.*
Journal of Water Process Engineering (Internet), 50, p.103289_1 - 103289_16, 2022/12
被引用回数:13 パーセンタイル:91.35(Engineering, Environmental)Due to synthesis variation affecting various graphene oxide (GO) physicochemical parameters and cost efficiency aspects, the present study investigated the influence of GO precursor components for GO precipitated nZVI nanocomposite (nZVI/GO) and optimized removal conditions to remove chloramphenicol (CAP) from water. In order to synthesize nZVI/GO nanocomposites, four methods of GO precursor synthesis were used, denoted GO1, GO2, GO3, and GO4. A novel synthesis process is introduced based on economic and time-less-consuming protocols to produce GO precursor. A series of desorption experiments were also implemented in various eluents to clarify the CAP removal mechanism. Interestingly, this study demonstrated the substantial impact of GO precursor on the nanocomposite performance in eliminating CAP. The introduced novel GO successfully served as an excellent nZVI precipitation medium and enhanced CAP removal efficiency. Empirical optimization demonstrated that nZVI/GO4-1:1 could eliminate up to 91% of 100 mg/L CAP by dosage as low as 0.25 g/L at pH 5. nZVI/GO4 displayed CAP removal stability throughout a more comprehensive pH range, and remarkable recyclability, making it more promising and practical than bare nZVI and other analyzed nanocomposites. Kinetics data demonstrated a high degree of compatibility with the pseudo-first-order (PFO) and pseudo-second-order (PSO). Through kinetics and statistical analyses, desorption experiments, FTIR spectroscopy, and EDX analysis, nZVI/GO4 removed some of the CAP through the adsorption mechanism controlled by physisorption and chemisorption. In contrast, the oxidation mechanism eliminated the remaining CAP.